

Select patients for the treatment of HR-positive, HER2-negative advanced or metastatic breast cancer with Alpelisib, based on the presence of one or more PIK3CA mutations in tumor tissue or plasma specimens. If no mutation is detected in a plasma specimen, test tumor tissue.
The recommended dose of Alpelisib is 300 mg (two 150 mg film-coated tablets) taken orally, once daily, with food.
Continue treatment until disease progression or unacceptable toxicity occurs.
Patients should take their dose of Alpelisib at approximately the same time each day.
Swallow Alpelisib tablets whole (tablets should not be chewed, crushed or split prior to swallowing). No tablet should be ingested if it is broken, cracked, or otherwise not intact.
If a dose of Alpelisib is missed, it can be taken with food within 9 hours after the time it is usually taken. After more than 9 hours, skip the dose for that day. The next day, take Alpelisib at the usual time.
If the patient vomits after taking the dose, advise the patient not to take an additional dose on that day, and to resume the dosing schedule the next day at the usual time.
When given with Alpelisib, the recommended dose of fulvestrant is 500 mg administered on Days 1, 15, and 29, and once monthly thereafter. Refer to the Full Prescribing Information for fulvestrant.
The recommended dose modifications for adverse reactions (ARs) are listed in Table 1.
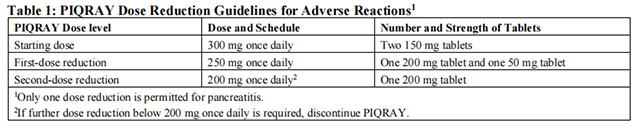
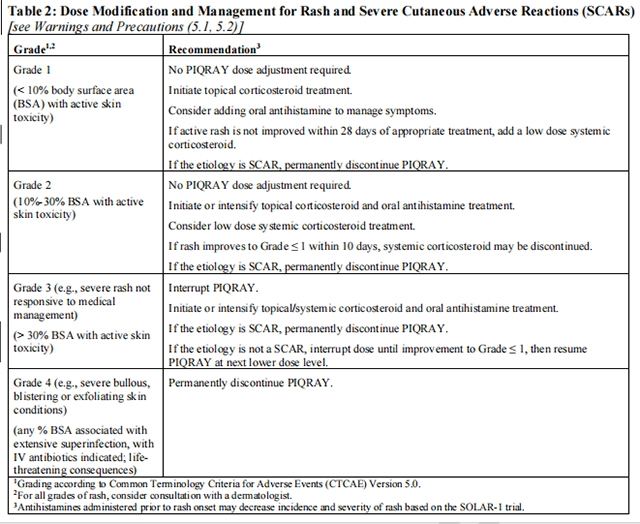
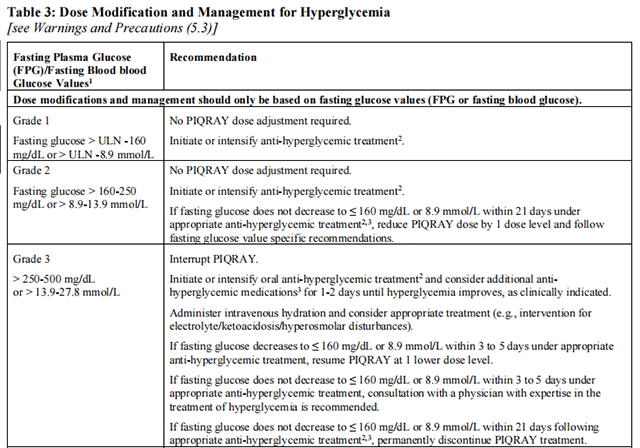
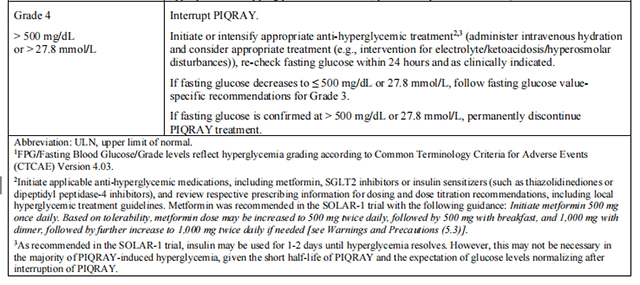
Tables 2, 3, 4, and 5 summarize recommendations for dose interruption, reduction, or discontinuation of Alpelisib in the management of specific ARs.
If a severe cutaneous adverse reaction (SCAR) is confirmed, permanently discontinue Alpelisib. Do not reintroduce Alpelisib in patients who have experienced previous SCAR during Alpelisib treatment.
Before initiating treatment with Alpelisib, test fasting plasma glucose (FPG), HbA1c, and optimize blood glucose. After initiating treatment with Alpelisib, monitor fasting glucose (FPG or fasting blood glucose) at least once every week for the first 2 weeks, then at least once every 4 weeks, and as clinically indicated. Monitor HbA1c every 3 months and as clinically indicated. In patients with risk factors for hyperglycemia, monitor fasting glucose more closely and as clinically indicated.
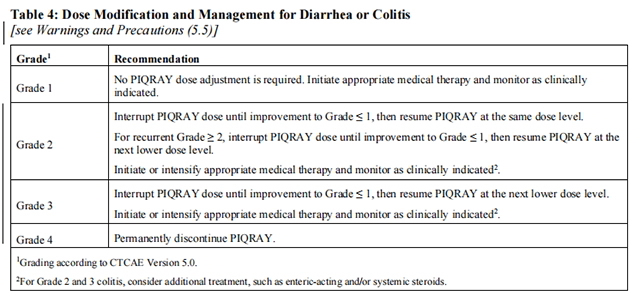
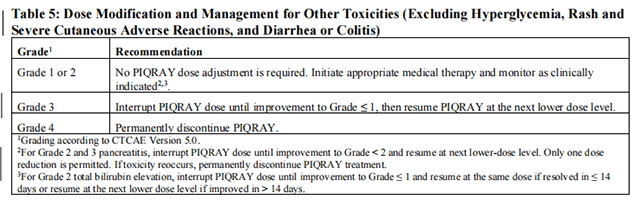
Refer to the Full Prescribing Information of fulvestrant for dose modification guidelines in the event of toxicity and for other relevant safety information.
from FDA,2022.11
Apelix is a common drug that is widely used in a variety of health management fields. Knowing the right dosage can ensure that your medication is working as well as it should. The ···【more】
Article source:Lucius LaosRelease date:2024-12-19Recommended:168
Apelix is a commonly used drug that is widely used in a variety of health management fields. Proper use can help patients better achieve their treatment goals. Understanding how Ap···【more】
Article source:Lucius LaosRelease date:2024-12-19Recommended:148
Alpelisib is the first PI3Kα inhibitor to be used in combination with fulvestrant for the treatment of HR/HER2- breast cancer. By targeting the PIK3CA mutation, Alpelisib significa···【more】
Article source:Lucius LaosRelease date:2024-08-26Recommended:290
Alpelisib has demonstrated a unique efficacy against PIK3CA mutations in HR/HER2- advanced breast cancer, and the combination with fulvestrant further enhances the anti-tumor effec···【more】
Article source:Lucius LaosRelease date:2024-08-23Recommended:220
Patients with HR/HER2- advanced breast cancer harboring PIK3CA mutations were significantly delayed in disease progression under the combination of Alpelisib and fulvestrant.What i···【more】
Article source:Lucius LaosRelease date:2024-08-23Recommended:303

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
