

• Tofacitinib (tofacitinib extended-release tablets) is not interchangeable or substitutable with Tofacitinib Oral Solution.
• Changes between Tofacitinib and Tofacitinib should be made by the healthcare provider.
• Do not initiate Tofacitinib Oral Solution in patients with an absolute lymphocyte count less than 500 cells/mm3 , an absolute neutrophil count (ANC) less than 1000 cells/mm3 or who have hemoglobin levels less than 9 g/dL.
• Dose interruption is recommended for management of lymphopenia, neutropenia, and anemia.
• Interrupt use of Tofacitinib Oral Solution if a patient develops a serious infection until the infection is controlled.
• Take Tofacitinib Oral Solution with or without food.
• Swallow Tofacitinib tablets whole and intact. Do not crush, split, or chew.
Table 1 displays the recommended adult daily dosage of Tofacitinib and Tofacitinib and dosage adjustments for patients receiving CYP2C19 and/or CYP3A4 inhibitors, in patients with moderate or severe renal impairment (including but not limited to those with severe insufficiency who are undergoing hemodialysis) or moderate hepatic impairment, with lymphopenia, neutropenia, or anemia.
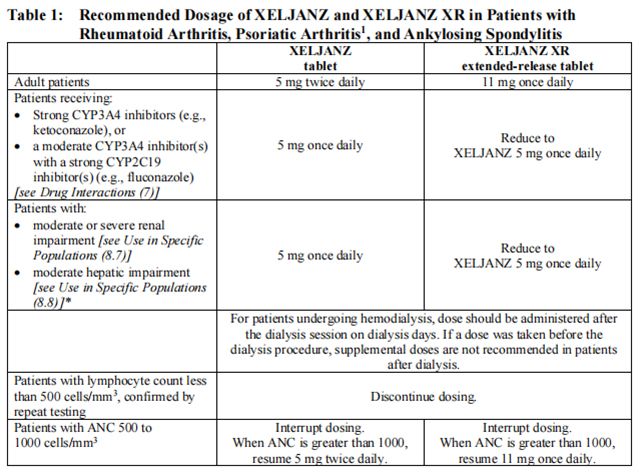
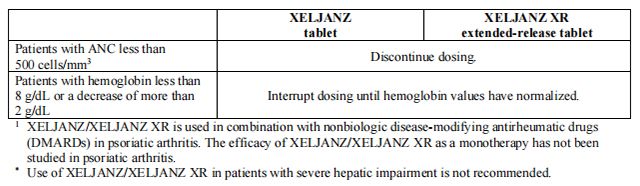
Patients treated with Tofacitinib tablets 5 mg twice daily may be switched to Tofacitinib extended-release tablets 11 mg once daily the day following the last dose of Tofacitinib 5 mg.
Table 2 displays the recommended adult daily dosage of Tofacitinib/Tofacitinib and dosage adjustments for patients receiving CYP2C19 and/or CYP3A4 inhibitors, with moderate or severe renal impairment (including but not limited to those with severe insufficiency who are undergoing hemodialysis) or moderate hepatic impairment, with lymphopenia, neutropenia or anemia.
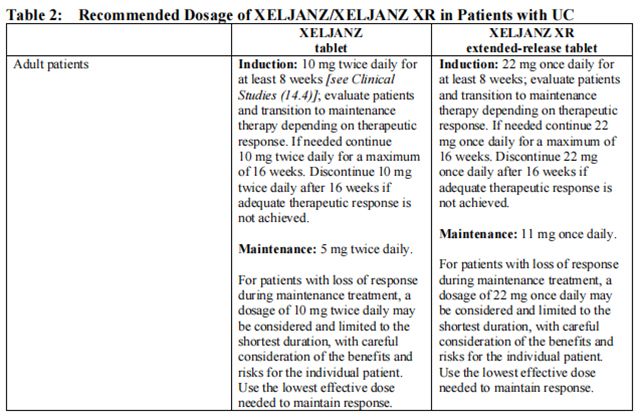
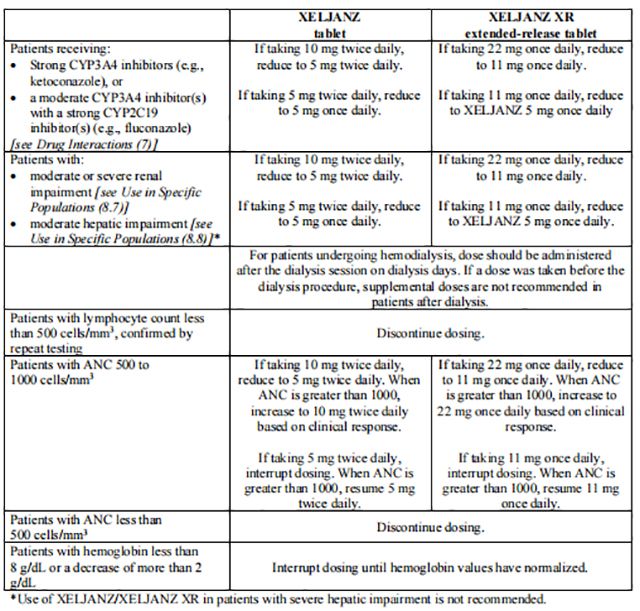
Patients treated with Tofacitinib 5 mg tablets twice daily may be switched to Tofacitinib extended-release tablets 11 mg once daily the day following the last dose of Tofacitinib tablets 5 mg. Patients treated with Tofacitinib 10 mg tablets twice daily may be switched to Tofacitinib extended-release tablets 22 mg once daily the day following the last dose of Tofacitinib 10 mg.
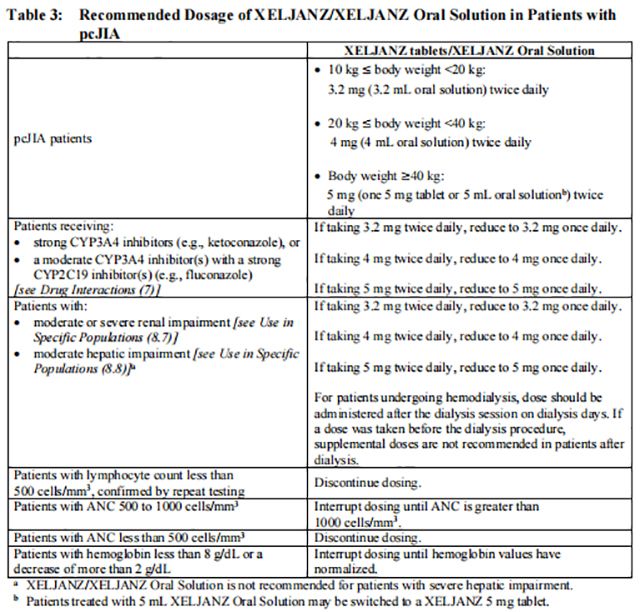
Table 3 displays the recommended body weight-based dosages for Tofacitinib tablets/Tofacitinib Oral Solution and dosage adjustments for patients receiving CYP2C19 and/or CYP3A4 inhibitors, in patients with moderate or severe renal impairment, including but not limited to those undergoing hemodialysis, with moderate hepatic impairment, with lymphopenia, neutropenia, or anemia.
Administer Tofacitinib Oral Solution using the included press-in bottle adapter and oral dosing syringe.
from FDA,2024.09

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
