

The recommended dosage of Tivozanib is 1.34 mg taken orally once daily for 21 days on treatment followed by 7 days off treatment for a 28-day cycle.
Continue treatment until disease progression or until unacceptable toxicity occurs.
Take Tivozanib with or without food. Swallow the Tivozanib capsule whole with a glass of water. Do not open the capsule.
If a dose is missed, the next dose should be taken at the next scheduled time. Do not take two doses at the same time.
Initiate medical management for diarrhea, nausea, or vomiting prior to dose interruption or reduction. If dose modifications are required for adverse reactions, reduce the dosage of Tivozanib to 0.89 mg for 21 days on treatment followed by 7 days off treatment for a 28-day cycle. Recommendations for dosage modifications are provided in Table 1.
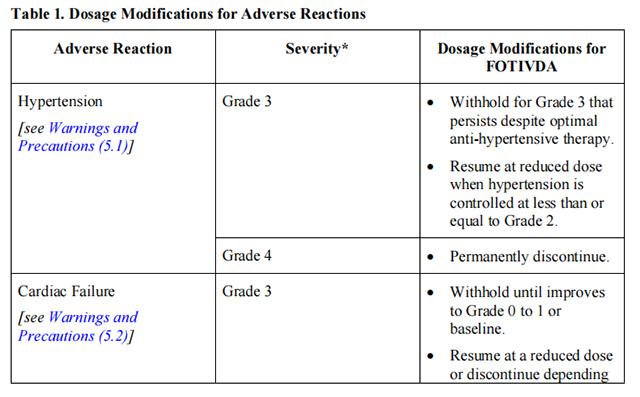
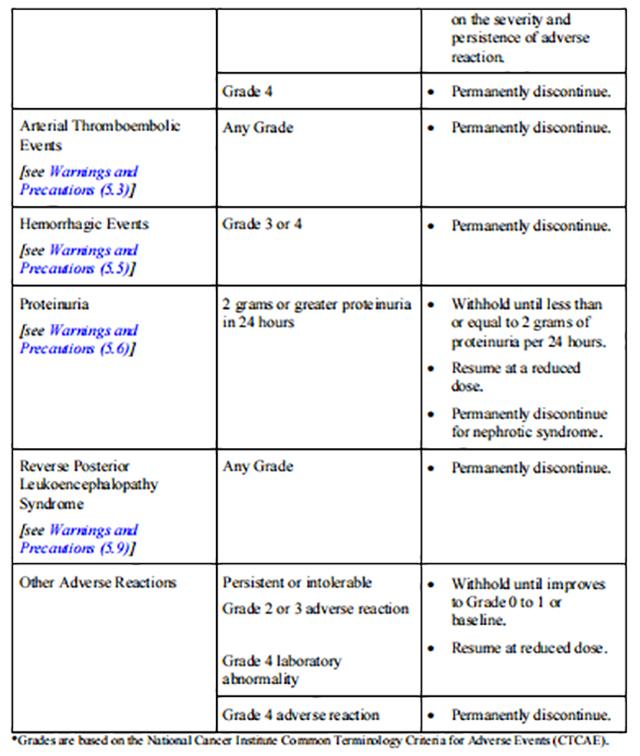
Reduce the recommended dosage of Tivozanib to 0.89 mg capsule taken orally once daily for 21 days on treatment followed by 7 days off treatment for a 28-day cycle for patients with moderate hepatic impairment.
from FDA,2021.03

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
