

Ribociclib can be taken with or without food.
Pre/perimenopausal women, or men, treated with the combination Ribociclib plus an aromatase inhibitor or fulvestrant, should be treated with a luteinizing hormone-releasing hormone (LHRH) agonist according to current clinical practice standards.
Patients should take their dose of Ribociclib at approximately the same time each day, preferably in the morning.
If the patient vomits after taking the dose, or misses a dose, no additional dose should be taken that day. The next prescribed dose should be taken at the usual time. Ribociclib tablets should be swallowed whole (tablets should not be chewed, crushed or split prior to swallowing). No tablet should be ingested if it is broken, cracked, or otherwise not intact.
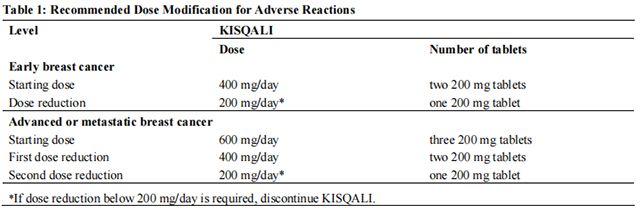
The recommended dosage of Ribociclib is 400 mg (two 200 mg film-coated tablets) taken orally, once daily for 21 consecutive days followed by 7 days off in 28-day treatment cycles. Ribociclib should be given in combination with an aromatase inhibitor. Refer to the Full Prescribing Information for the recommended dosage of the aromatase inhibitor.
In patients with early breast cancer, treatment with Ribociclib should continue for 3 years or until disease recurrence or unacceptable toxicity occurs.
The recommended dosage of Ribociclib is 600 mg (three 200 mg film-coated tablets) taken orally, once daily for 21 consecutive days followed by 7 days off in 28-day treatment cycles. Ribociclib should be given in combination with endocrine therapy (fulvestrant or an aromatase inhibitor). Refer to the Full Prescribing Information for the recommended dose of endocrine therapy.
The recommended dose modifications for adverse reactions are listed in Table 1.
Tables 2, 3, 4, 5, 6, and 7 summarize recommendations for dose interruption, reduction, or discontinuation of Ribociclib in the management of specific adverse reactions. Dose modification of Ribociclib is recommended based on individual patient safety and tolerability.
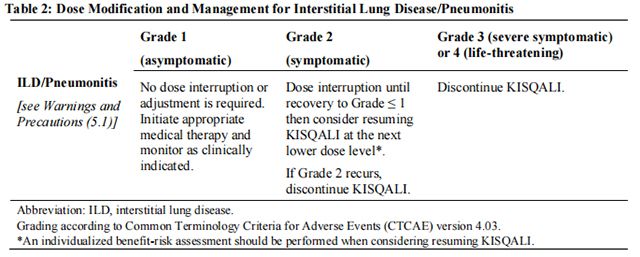
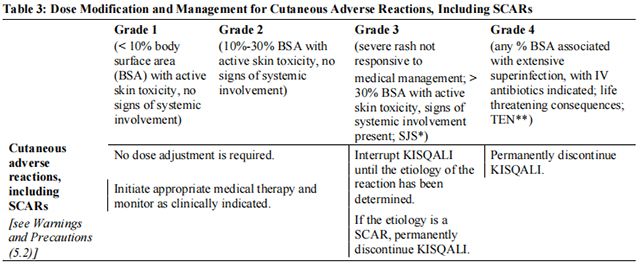
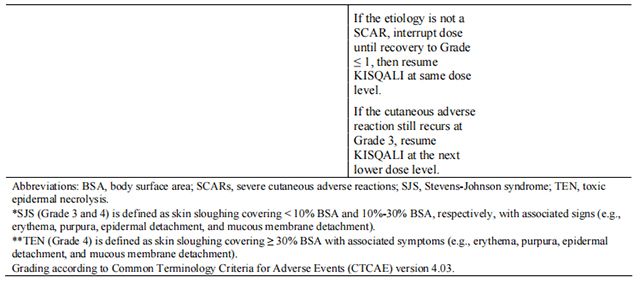
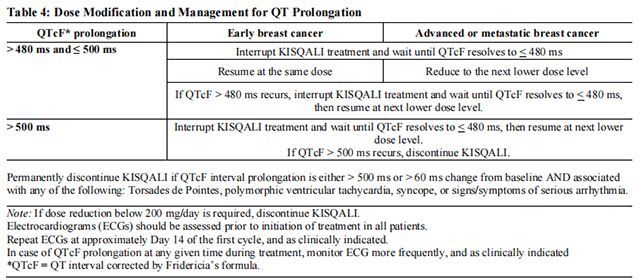
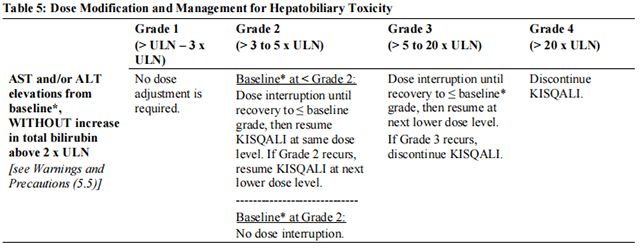
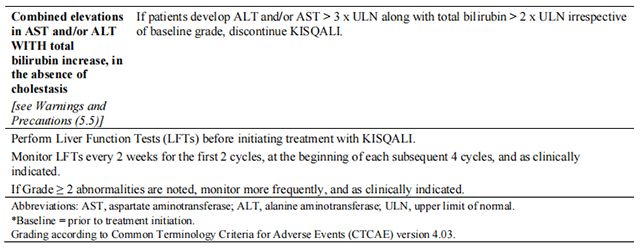
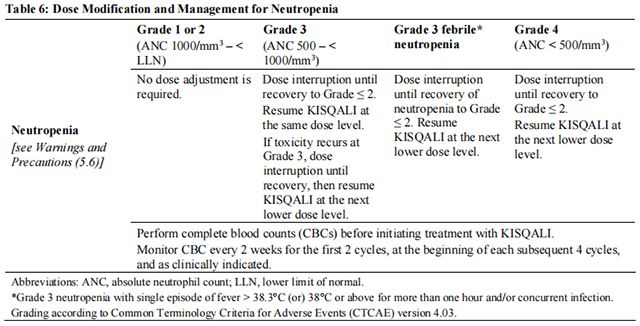
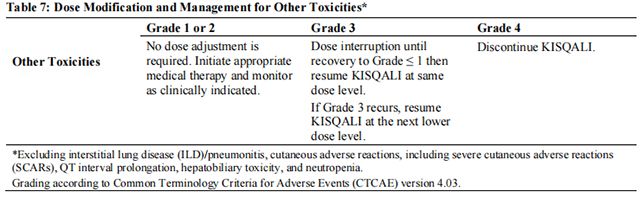
Refer to the Full Prescribing Information for the coadministered aromatase inhibitor or fulvestrant for dose modification guidelines in the event of toxicity and other relevant safety information.
Avoid concomitant use of Ribociclib with strong CYP3A inhibitors and consider an alternative concomitant medication with less potential for CYP3A inhibition.
If a strong CYP3A inhibitor must be coadministered, reduce the Ribociclib dose as shown in Table 8.

If the strong inhibitor is discontinued, change the Ribociclib dose (after at least 5 half-lives of the strong CYP3A inhibitor) to the dose used prior to the initiation of the strong CYP3A inhibitor.
The recommended dose modifications for patients with hepatic impairment are shown in Table 9.
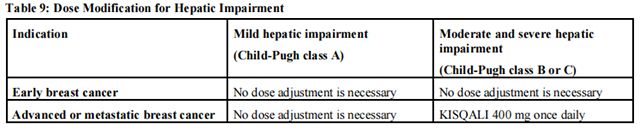
Review the Full Prescribing Information for the co-administered aromatase inhibitor or fulvestrant for dose modifications related to hepatic impairment.
The recommended starting dose is 200 mg Ribociclib once daily for patients with severe renal impairment.
from FDA,2024.09

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
