

Select patients with resectable tumors for the adjuvant treatment of NSCLC with Osimertinib based on the presence of EGFR exon 19 deletions or exon 21 L858R mutations in tumor specimens.
Select patients for the first-line treatment of metastatic EGFR-positive NSCLC with Osimertinib based on the presence of EGFR exon 19 deletions or exon 21 L858R mutations in tumor or plasma specimens. If these mutations are not detected in a plasma specimen, test tumor tissue if feasible.
Select patients for the treatment of metastatic EGFR T790M mutation-positive NSCLC with Osimertinib following progression on or after EGFR TKI therapy based on the presence of an EGFR T790M mutation in tumor or plasma specimens.Testing for the presence of the T790M mutation in plasma specimens is recommended only in patients for whom a tumor biopsy cannot be obtained. If this mutation is not detected in a plasma specimen, re-evaluate the feasibility of biopsy for tumor tissue testing.
The recommended dosage of Osimertinib is 80 mg tablet once a day. Osimertinib can be taken with or without food.
If a dose of Osimertinib is missed, do not make up the missed dose and take the next dose as scheduled.
Treat patients in the adjuvant setting until disease recurrence, or unacceptable toxicity, or for up to 3 years.
Treat patients with metastatic lung cancer until disease progression or unacceptable toxicity.
Disperse tablet in 60 mL (2 ounces) of non-carbonated water only. Stir until tablet is dispersed into small pieces (the tablet will not completely dissolve) and swallow immediately. Do not crush, heat, or ultrasonicate during preparation. Rinse the container with 120 mL to 240 mL (4 to 8 ounces) of water and immediately drink.
If administration via nasogastric tube is required, disperse the tablet as above in 15 mL of non-carbonated water, and then use an additional 15 mL of water to transfer any residues to the syringe. The resulting 30 mL liquid should be administered as per the nasogastric tube instructions with appropriate water flushes (approximately 30 mL).
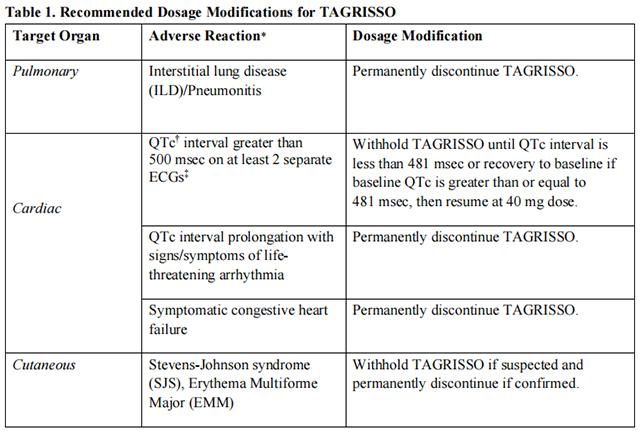
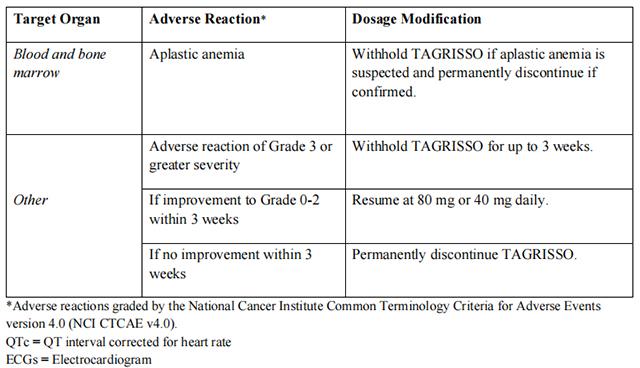
Strong CYP3A4 Inducers
If concurrent use is unavoidable, increase Osimertinib dosage to 160 mg daily when co-administering with a strong CYP3A inducer. Resume Osimertinib at 80 mg 3 weeks after discontinuation of the strong CYP3A4 inducer.
from FDA,2022.10
To explore the proper use of osimertinib and its role in the treatment of lung cancer.What is the dosage of osimertinibKnowing the correct dosage of osimertinib is essential to imp···【more】
Article source:Lucius LaosRelease date:2025-03-05Recommended:225
To explore new advances in the treatment of lung cancer, especially effective therapies targeting specific gene mutations.How to use osimertinibKnowing how to properly use osimerti···【more】
Article source:Lucius LaosRelease date:2025-03-04Recommended:276
Osimertinib is a third-generation EGFR-TKI-targeted drug, and its clinical application needs to strictly follow the dosage specifications and medication precautions to ensure the t···【more】
Article source:Lucius LaosRelease date:2025-03-04Recommended:240

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
