

The efficacy of Koselugo was evaluated in SPRINT Phase II Stratum 1, an open-label, multicenter, single arm trial (NCT01362803). Eligible patients were required to have NF1 with inoperable PN, defined as a PN that could not be completely removed without risk for substantial morbidity due to encasement of, or close proximity to, vital structures, invasiveness, or high vascularity of the PN. Patients were also required to have significant morbidity related to the target PN. Morbidities that were present in > 20% of patients included disfigurement, motor dysfunction, pain, airway dysfunction, visual impairment, and bladder/bowel dysfunction. Patients received Koselugo 25 mg/m2 orally twice daily until disease progression or unacceptable toxicity.
The major efficacy outcome measure was overall response rate (ORR), defined as the percentage of patients with complete response (defined as disappearance of the target PN) or confirmed partial response (defined as ≥ 20% reduction in PN volume confirmed at a subsequent tumor assessment within 3-6 months). The target PN, defined as the PN that caused relevant clinical symptoms or complications (PN-related morbidities), was evaluated for response rate using centrally read volumetric magnetic resonance imaging (MRI) analysis per Response Evaluation in Neurofibromatosis and Schwannomatosis (REiNS) criteria. Tumor response was evaluated at baseline and while on treatment after every 4 cycles for 2 years, and then every 6 cycles. An additional efficacy outcome measure was duration of response (DoR).
A total of 50 pediatric patients received Koselugo. The median age was 10.2 years (range 3.5 to 17.4 years); 60% were male; and 84% were White, 8% were Black and 2% were Asian.
Efficacy results are provided in Table 8. The median time to onset of response was 7.2 months (range: 3.3 months to 1.6 years).
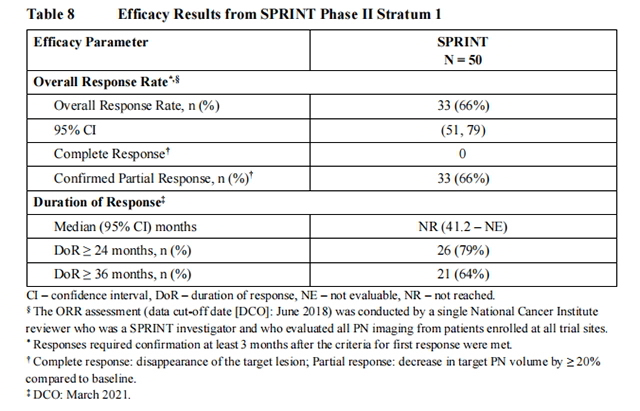
An independent centralized review of tumor response per REiNS criteria (data cut-off June 2018) resulted in an ORR of 44% (95% CI: 30, 59).
from FDA,2024.01
Koselugois a precision small molecule MEK blocker engineered for specific tumors, particularly in the treatment of neurofibromatosis (NF1), demonstrating superior performance.Does ···【more】
Article source:Lucius LaosRelease date:2024-08-22Recommended:235
Koselugois a precision small molecule MEK inhibitor designed for specific tumor types, especially in the treatment of neurofibromatosis (NF1).Is Koselugoeffective?The therapeutic e···【more】
Article source:Lucius LaosRelease date:2024-08-22Recommended:292
Koselugo is a carefully developed small molecule MEK blocker (also known as a mitogen activation inhibitor) whose core medical strategy focuses on precisely targeting targeted inte···【more】
Article source:Lucius LaosRelease date:2024-08-22Recommended:281

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
