

TAS-120-101 (NCT02052778), a multicenter, open-label, single-arm trial, evaluated the efficacy of Futibatinib in 103 patients with previously treated, unresectable, locally advanced or metastatic intrahepatic cholangiocarcinoma.The presence of FGFR2 fusions or other rearrangements was determined in 102 enrolled patients (99%) using next generation sequencing (NGS) testing. Qualifying in-frame fusions and other rearrangements were predicted to have a breakpoint within intron 17/exon 18 of the FGFR2 gene leaving the FGFR2 kinase domain intact.
Patients received Futibatinib at a dosage of 20 mg orally once daily until disease progression or unacceptable toxicity. The major efficacy outcome measures were overall response rate (ORR) and duration of response (DoR) as determined by an independent review committee (IRC) according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1.
The trial population characteristics were: Median age was 58 years (range: 22 to 79 years) with 22% of patients ≥65 years, 56% were female, race was: 50% White, 29% Asian, 8% Black or African American, 1% Native Hawaiian or Other Pacific Islander, 13% unknown, baseline Eastern Cooperative Oncology Group (ECOG) performance status of 0 (47%) or 1 (53%). Seventy-eight percent (78%) of patients had in-frame FGFR2 gene fusions and the most commonly identified FGFR2 fusion partner was BICC1 (n=24, 23%). Twenty-two percent (22%) of patients had other FGFR2 rearrangements that may not be in-frame with the partner gene or the partner gene was not identifiable.
All patients had received at least 1 prior systemic therapy, 30% had 2 prior lines of therapy, and 23% had 3 or more prior lines of therapy. All patients received a prior platinum-based therapy including 91% with prior gemcitabine/cisplatin.
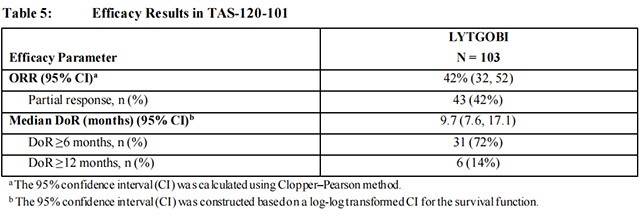
Efficacy results are summarized in Table 5. The median time to response was 2.5 months (range 0.7 – 7.4 months).
FDA,2022.09

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
