

Stop treatment with Cabozantinib at least 3 weeks prior to scheduled surgery, including dental surgery.
Do not substitute Cabozantinib tablets with cabozantinib capsules.
The recommended dosage of Cabozantinib as a single agent is 60 mg once daily until disease progression or unacceptable toxicity administered as recommended.

The recommended dosage of Cabozantinib as a single agent is 60 mg once daily until disease progression or unacceptable toxicity administered as recommended.
The recommended dosage of Cabozantinib as a single agent for adult and pediatric patients 12 years of age and older with BSA greater than or equal to 1.2 ㎡ is 60 mg once daily until disease progression or unacceptable toxicity administered as recommended.
The recommended dosage of Cabozantinib as a single agent in pediatric patients 12 years of age and older with BSA less than 1.2 ㎡ is 40 mg once daily until disease progression or unacceptable toxicity administered as recommended.
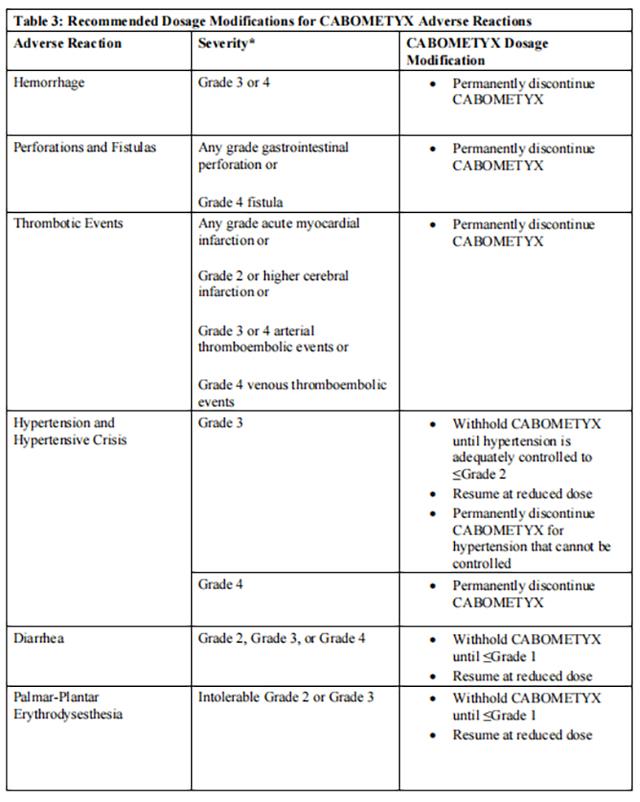
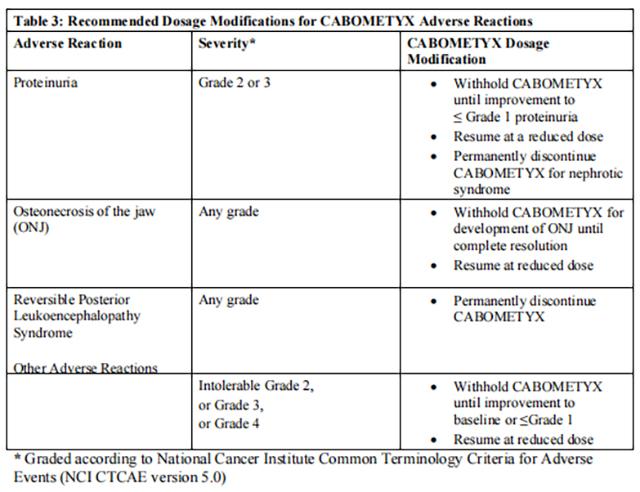
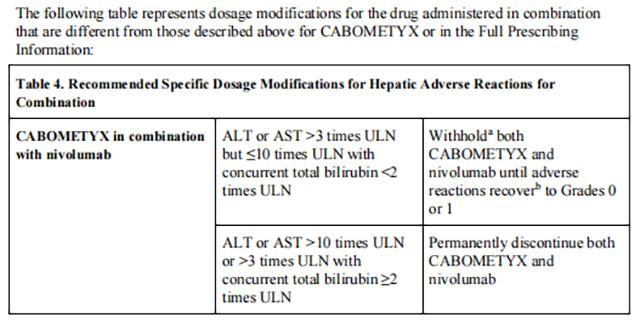
Withhold Cabozantinib for:
• Intolerable Grade 2 adverse reactions
• Grade 3 or 4 adverse reactions
• Osteonecrosis of the jaw
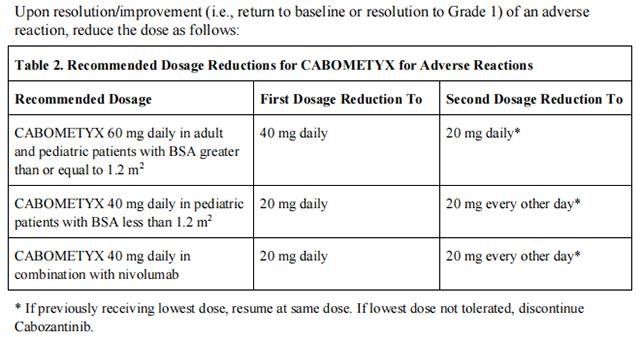
Reduce the daily Cabozantinib dose by 20 mg (for example, from 60 mg to 40 mg daily or from 40 mg to 20 mg daily or from 20 mg daily to 20 mg every other day in pediatric patients with BSA less than 1.2 m2 ). Resume the dose that was used prior to initiating the strong CYP3A4 inhibitor 2 to 3 days after discontinuation of the strong inhibitor.
Increase the daily Cabozantinib dose by 20 mg (for example, from 60 mg to 80 mg daily or from 40 mg to 60 mg daily) as tolerated. Resume the dose that was used prior to initiating the strong CYP3A4 inducer 2 to 3 days after discontinuation of the strong inducer. Do not exceed a daily dose of 80 mg.
Reduce the starting dose of Cabozantinib 60 mg daily to 40 mg daily or 40 mg daily to 20 mg daily (for pediatric patients with BSA less than 1.2 m2 ) in patients with moderate hepatic impairment (Child-Pugh B).
Do not administer Cabozantinib with food. Administer at least 1 hour before or at least 2 hours after eating.
• Swallow Cabozantinib tablets whole. Do not crush Cabozantinib tablets.
• Do not take a missed dose within 12 hours of the next dose.
• Modify the Cabozantinib dose for patients taking drugs known to strongly induce or inhibit CYP3A4 and for patients with moderate hepatic impairment.
from FDA,2023.09
Cabozantinib is a multi-kinase inhibitor with a wide range of clinical applications. This article will discuss the dosage of cabozantinib and its associated factors, as well as the···【more】
Article source:Lucius LaosRelease date:2025-02-18Recommended:271
Cabozantinib is a multi-targeted tyrosine kinase inhibitor that plays an important role in clinical treatment. This article will detail the use of cabozantinib and its related cons···【more】
Article source:Lucius LaosRelease date:2025-02-18Recommended:280

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
