

Study BLC2001 (NCT02365597) was a multicenter, open-label, single-arm study to evaluate the efficacy and safety of BALVERSA in patients with locally advanced or metastatic urothelial carcinoma (mUC). FGFR mutation status for screening and enrollment of patients was determined by a clinical trial assay (CTA). The efficacy population consists of a cohort of eighty-seven patients who were enrolled in this study with disease that had progressed on or after at least one prior chemotherapy and that had at least 1 of the following genetic alterations: FGFR3 gene mutations (R248C, S249C, G370C, Y373C) or FGFR gene fusions (FGFR3-TACC3, FGFR3-BAIAP2L1, FGFR2-BICC1, FGFR2-CASP7), as determined by the CTA performed at a central laboratory. Tumor samples from 69 patients were tested retrospectively by the QIAGEN therascreen® FGFR RGQ RT-PCR Kit, which is the FDA-approved test for selection of patients with mUC for BALVERSA.
Patients received a starting dose of BALVERSA at 8 mg once daily with a dose increase to 9 mg once daily in patients whose serum phosphate levels were below the target of 5.5 mg/dL between days 14 and 17; a dose increase occurred in 41% of patients. BALVERSA was administered until disease progression or unacceptable toxicity. The major efficacy outcome measures were ORR and duration of response (DoR), as determined by blinded independent review committee (BIRC) according to RECIST v1.1.
The median age was 67 years (range: 36 to 87 years), 79% were male, and 74% were Caucasian. Most patients (92%) had a baseline Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Sixty-six percent of patients had visceral metastases. Eighty-four (97%) patients received at least one of cisplatin or carboplatin previously. Fifty-six percent of patients only received prior cisplatin-based regimens, 29% received only prior carboplatin-based regimens, and 10% received both cisplatin and carboplatin-based regimens. Three (3%) patients had disease progression following prior platinum-containing neoadjuvant or adjuvant therapy only. Twenty-four percent of patients had been treated with prior anti PD-L1/PD-1 therapy.
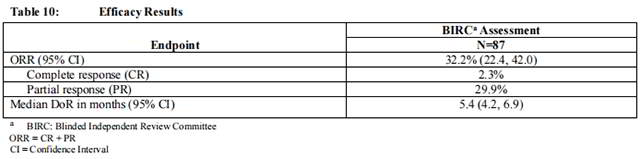
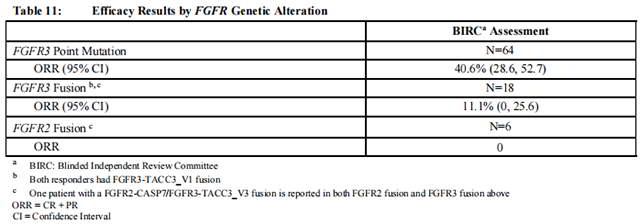
Efficacy results are summarized in Table 10 and Table 11. ORR was 32.2%. Responders included patients who had previously not responded to anti PD-L1/PD-1 therapy.
from FDA,2024.01
1. Targeted therapySpecific inhibitionAs a tyrosine kinase inhibitor, balversa specifically targets the FGFR (fibroblast growth factor receptor) signaling pathway. FGFR is abnormal···【more】
Article source:Lucius LaosRelease date:2024-11-12Recommended:254
Balversais an oral anti-cancer star targeting FGFR mutations, which has a significant inhibitory effect on tumors such as urothelial carcinoma, but it has not yet landed in the dom···【more】
Article source:Lucius LaosRelease date:2024-08-27Recommended:293
Balversa is an oral anticancer drug that specializes in FGFR mutant tumors, such as urothelial carcinoma, and effectively inhibits tumor proliferation, but has not yet been introdu···【more】
Article source:Lucius LaosRelease date:2024-08-27Recommended:281
Balversa is an oral anticancer drug used for the treatment of locally advanced or metastatic urothelial carcinoma, inhibiting FGFR activity to stop tumor growth, but it is not curr···【more】
Article source:Lucius LaosRelease date:2024-08-27Recommended:235

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
