

Select patients for the treatment of locally advanced or metastatic urothelial carcinoma with Balversa based on the presence of susceptible FGFR3 genetic alterations in tumor specimens as detected by an FDA-approved companion diagnostic.
The recommended starting dose of Balversa is 8 mg (two 4 mg tablets) orally once daily, with a dose increase to 9 mg (three 3 mg tablets) once daily based on tolerability, including hyperphosphatemia, at 14 to 21 days.
Swallow tablets whole with or without food. If vomiting occurs any time after taking Balversa, the next dose should be taken the next day. Treatment should continue until disease progression or unacceptable toxicity occurs.
If a dose of Balversa is missed, it can be taken as soon as possible on the same day. Resume the regular daily dose schedule for Balversa the next day. Extra tablets should not be taken to make up for the missed dose.
Assess serum phosphate levels 14 to 21 days after initiating treatment. Increase the dose of Balversa to 9 mg once daily if serum phosphate level is < 9.0 mg/dL and there are no ocular disorders or Grade 2 or greater adverse reactions. Monitor phosphate levels monthly for hyperphosphatemia.
The recommended dose modifications for adverse reactions are listed in Table 1.
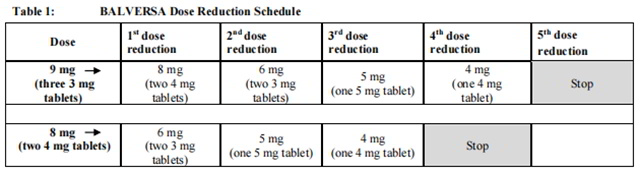
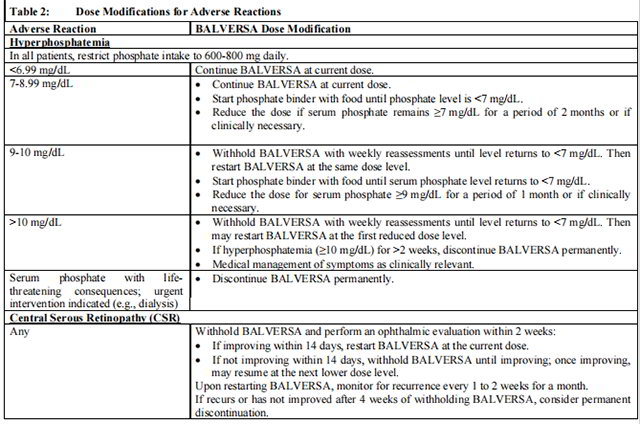
Table 2 summarizes recommendations for dose interruption, reduction, or discontinuation of Balversa in the management of specific adverse reactions.

from FDA,2024.01
Accurately understanding the dosage of Balversa is the key to achieving the best treatment results. Below, let's discuss the dosage of Balversa in detail.What is the dosage of ···【more】
Article source:Lucius LaosRelease date:2024-08-27Recommended:189
Mastering the correct use of Balversais the first step to ensuring efficacy and safety, so let's dive into its detailed medication guidelines.How to use BalversaThe use of Balv···【more】
Article source:Lucius LaosRelease date:2024-08-27Recommended:208
In-depth analysis of the treatment of Balversa, the primary concern is its recommended dose, which is directly related to the safety and effect of treatment.What is the recommended···【more】
Article source:Lucius LaosRelease date:2024-08-27Recommended:276

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
