

The efficacy of Alectinib for the adjuvant treatment of patients with ALK-positive NSCLC following complete tumor resection was evaluated in a global, randomized open-label clinical trial (ALINA: NCT03456076).
ALINA demonstrated a statistically significant improvement in DFS for patients treated with Alectinib compared to patients treated with chemotherapy. OS data were not mature at the time of DFS analysis with 2.3% of deaths reported in the ITT population.
The efficacy results from ALINA are summarized in Table 10 and Figure 1.
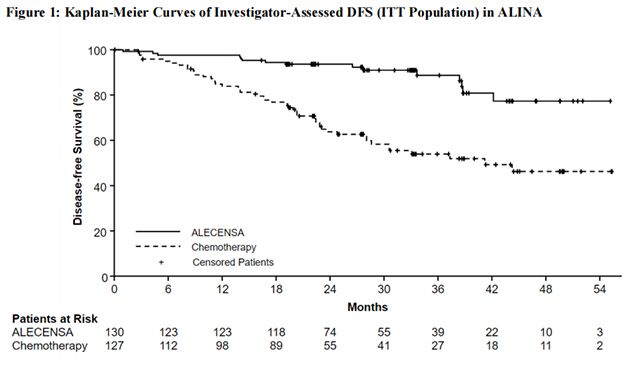
In an exploratory analysis of site(s) of relapse, the proportion of patients with brain involvement at the time of disease recurrence was 4 patients (3.1%) in the Alectinib arm and 14 patients (11%) in the chemotherapy arm.
from FDA,2024.04

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
