

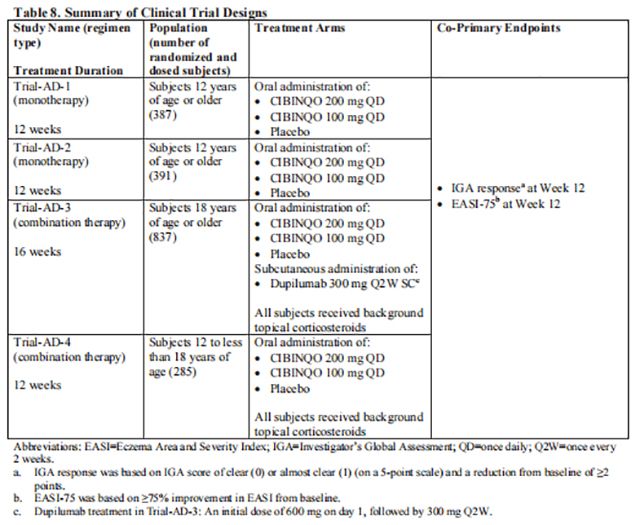
The efficacy of Cibinqo as monotherapy and in combination with background topical corticosteroids was evaluated in 4 randomized, double-blind, placebo-controlled trials [Trial-AD-1 (NCT03349060), Trial-AD-2 (NCT03575871), Trial-AD-3 (NCT03720470), and Trial-AD-4 (NCT03796676)] in 1900 subjects (see Table 8). Trial-AD-1 and Trial-AD-2 enrolled adult and pediatric subjects 12 years of age and older.
Trial-AD-1, Trial-AD-2, Trial-AD-3, and Trial-AD-4 assessed the co-primary endpoints of IGA and EASI-75 responses at Week 12. The designs of the trials are summarized in Table 8.
The results of the Cibinqo monotherapy trials (Trial-AD-1 and Trial-AD-2) are presented next.
The proportion of subjects achieving PP-NRS4 at Week 2 (defined as an improvement of-4 points from baseline in PP-NRS) was higher in subjects treated with Cibinqo monotherapy 200 mg once daily (28% in Trial-AD-1 and 24% in Trial-AD-2) and 100 mg once daily (11% in both trials) compared to placebo (2% in both trials).
A higher proportion of subjects in the Cibinqo monotherapy 100 mg or 200 mg once daily arms compared to placebo achieved improvement in itching at Week 12.
The results of Cibinqo in combination with background topical corticosteroids in subjects 18 years of age and older (Trial-AD-3) are presented in next.
The proportions of subjects achieving PP-NRS4 at Week 2 was higher in subjects treated with Cibinqo 200 mg once daily (30%) and 100 mg once daily (14%) in combination with background medicated topical therapies compared to placebo (8%).
The proportion of pediatric subjects 12 to less than 18 years of age achieving PP-NRS4 at Week 2 in Trial-AD- 4 was higher with Cibinqo 200 mg once daily (25%) and 100 mg once daily (13%) compared to placebo (8%).
A higher proportion of subjects in the Cibinqo 200 mg once daily arm compared to placebo achieved improvement in itching at Week 12.
from FDA,2023.12
This article examines the performance of abuxitinib in the treatment of atopic dermatitis and its economic implications.How effective is the treatment of abuxitinib?It is important···【more】
Article source:Lucius LaosRelease date:2025-06-10Recommended:152
As an oral drug targeting specific immune responses, abuxitinib has attracted attention in clinical applications in recent years.Is abuxitinib effective?Its mechanism of action pro···【more】
Article source:Lucius LaosRelease date:2025-06-09Recommended:166
As a new type of therapeutic drug, aboxitinib has attracted attention in clinical application in recent years.How effective is the treatment of abuxitinib?Its mechanism of action a···【more】
Article source:Lucius LaosRelease date:2025-06-09Recommended:134

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
