

In vivo drug-drug interaction studies were not conducted with valganciclovir. However, because valganciclovir is rapidly and extensively converted to ganciclovir, drug-drug interactions associated with ganciclovir will be expected for Valcyte. Drug-drug interaction studies with ganciclovir were conducted in patients with normal renal function. Following concomitant administration of Valcyte and other renally excreted drugs, patients with impaired renal function may have increased concentrations of ganciclovir and the coadministered drug. Therefore, these patients should be closely monitored for toxicity of ganciclovir and the coadministered drug.
Established and other potentially significant drug interactions conducted with ganciclovir are listed in Table 9.
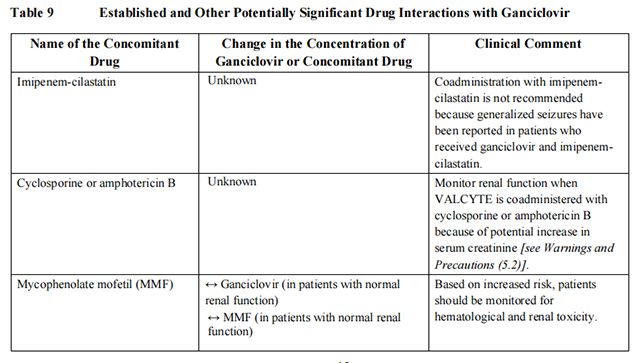
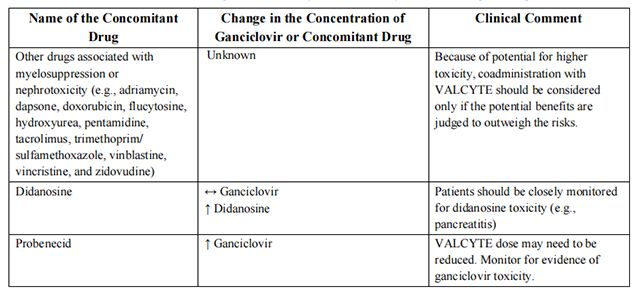
from FDA,2021.12

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
