

The effectiveness of Stiripentol for the treatment of seizures associated with Dravet syndrome was established in 2 multicenter placebo-controlled double-blind randomized studies (Study 1 and Study 2), conducted according to similar protocols. To be enrolled in either study, patients were required to be 3 years to less than 18 years of age, to have Dravet syndrome (ILAE classification of epilepsy, 1989), and to be inadequately controlled on clobazam and valproate, with at least 4 generalized clonic or tonic-clonic seizures per month despite optimized therapy.
Eligible patients were enrolled in a 1-month baseline period during which they continued to receive their optimized antiepileptic treatment. Following this 1-month baseline, patients were randomly allocated to receive either Stiripentol (fixed dose of 50 mg/kg/day in divided doses with no dose titration) or placebo, added to their treatment with clobazam and valproate. Duration of double-blind treatment was 2 months. The frequency of generalized clonic or tonic-clonic seizures during the study was recorded by patients and/or their caregivers, using a diary. Although patients with Dravet syndrome have several different types of seizures, only generalized clonic or tonic-clonic seizures were recorded, as other seizure types can be difficult to recognize by patients and/or their caregivers as seizures.
The primary efficacy endpoint for both studies was the responder rate. A responder was defined as a patient who experienced a greater than 50% decrease in the frequency (per 30 days) of generalized clonic or tonic-clonic seizures during the double-blind treatment period compared tothe 4-week baseline period (i.e., placebo run-in). The mean change from baseline in frequency of generalized clonic or tonic clonic seizures was also evaluated.
In Study 1 (n=41), 21 patients were randomized to Stiripentol, and 20 patients to placebo. In Study 2 (n=23), 12 patients were randomized to Stiripentol, and 11 patients to placebo. In both studies, the demographic and baseline clinical characteristics were similar between the treatment groups.
Table 5 summarizes the results of the primary endpoint for Stiripentol in each study.
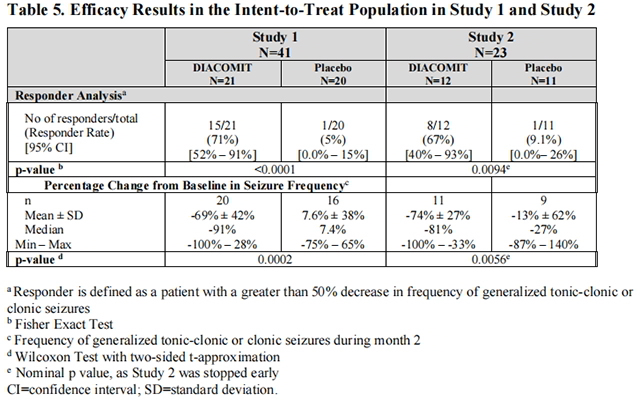
from FDA,2022.07

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
