

The effect of sparsentan on proteinuria was assessed in a randomized, double-blind, active-controlled, multicenter, global study (PROTECT, NCT03762850) in adults with biopsy-proven IgAN, eGFR ≥30 mL/min/1.73m2, and total urine protein ≥1.0 g/day on a maximized stable dose of RAS inhibitor treatment that was at least 50% of maximum labeled dose. Patients with other glomerulopathies or those who had been recently treated with systemic immunosuppressants were excluded.
The primary endpoint was the relative change from baseline in UPCR at Week 36 (Table 3). The mean percent change from baseline over time is displayed in Figure 1.
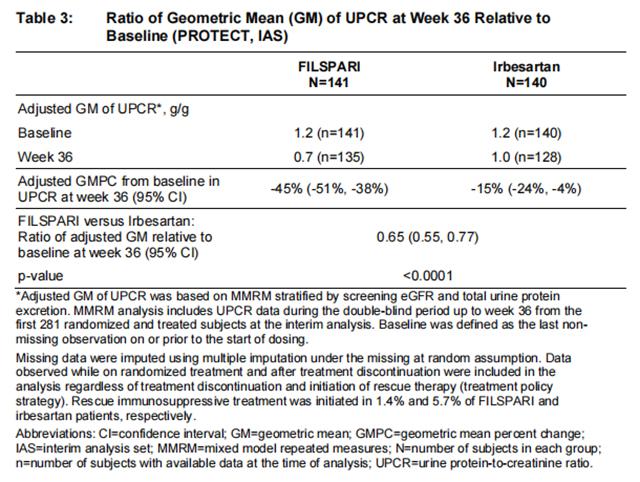
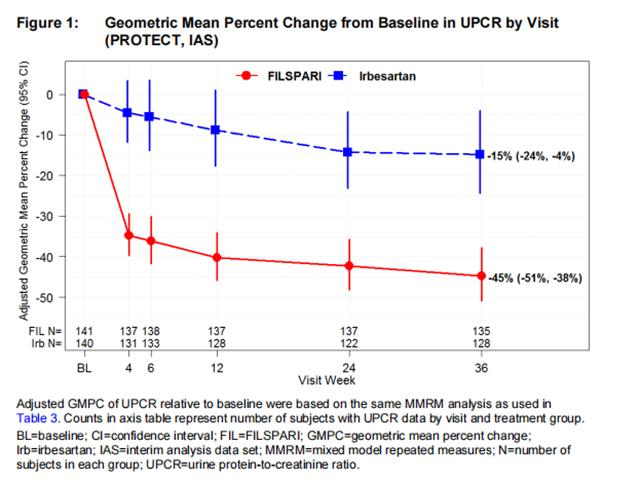
The treatment effect on UPCR at Week 36 was consistent across subgroups including age, sex, race, and baseline eGFR and proteinuria levels.
from FDA,2023.02

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
