

Prior to initiating treatment with sparsentan, discontinue use of renin-angiotensin-aldosterone system (RAAS) inhibitors, endothelin receptor antagonists (ERAs), and aliskiren.
Initiate treatment with sparsentan only after measuring aminotransferase levels and total bilirubin. Avoid initiation in patients with elevated aminotransferases (>3x ULN). Continue required monitoring monthly for the first 12 months after initiation or restarting following an interruption due to elevated transaminases, then every 3 months during treatment with sparsentan.
Initiate treatment with sparsentan in patients who can become pregnant only after confirmation of a negative pregnancy test. Pregnancy tests are required monthly during treatment and one month after discontinuation of treatment with sparsentan.
Initiate treatment with sparsentan at 200 mg orally once daily. After 14 days, increase to the recommended dose of 400 mg once daily, as tolerated. When resuming treatment with sparsentan after an interruption, consider titration of sparsentan, starting at 200 mg once daily. After 14 days, increase to the recommended dose of 400 mg once daily.
Instruct patient to swallow tablets whole with water prior to the morning or evening meal. Maintain the same dosing pattern in relationship to meals. If a dose is missed, take the next dose at the regularly scheduled time. Do not take double or extra doses.
If aminotransferase levels increase, adjust monitoring and treatment plan according to Table 1.
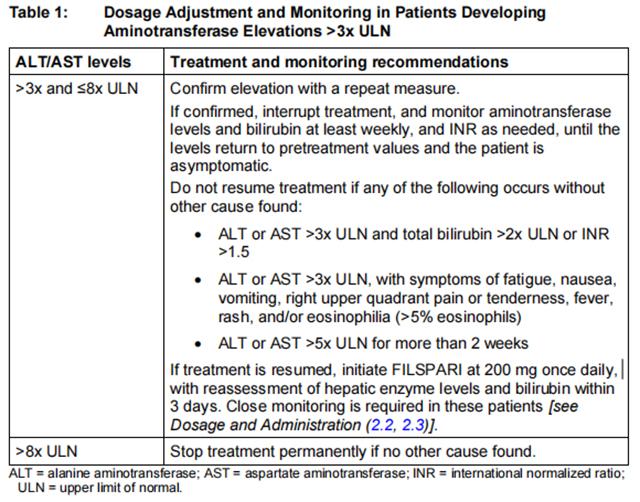
Do not resume treatment in patients who have experienced clinical symptoms of hepatotoxicity or in patients whose hepatic enzyme levels and bilirubin have not returned to pretreatment levels.
Avoid concomitant use of strong CYP3A inhibitors with sparsentan.
If a strong CYP3A inhibitor cannot be avoided, interrupt treatment with sparsentan.
from FDA,2023.02

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
