

Select patients for the treatment of locally advanced or metastatic cholangiocarcinoma with pemigatinib based on the presence of an FGFR2 fusion or rearrangement as detected by anFDA-approved test.
Take pemigatinib with or without food at approximately the same time every day.
Swallow tablets whole. Do not crush, chew, split, or dissolve tablets.If the patient misses a dose of pemigatinib by 4 or more hours or if vomiting occurs, resume dosing with the next scheduled dose.
The recommended dose reductions for adverse reactions are provided in Table 1.
Table 1: Recommended Dose Reductions for pemigatinib for Adverse Reactions
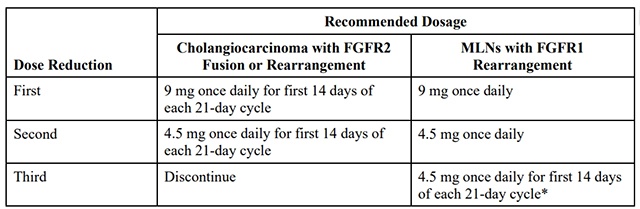
* Permanently discontinue pemigatinib if unable to tolerate 4.5 mg once daily for 14 days of each 21-day cycle.
The recommended dosage modifications for adverse reactions are provided in Table 2.
Table 2: Recommended Dosage Modifications for pemigatinib Adverse Reactions
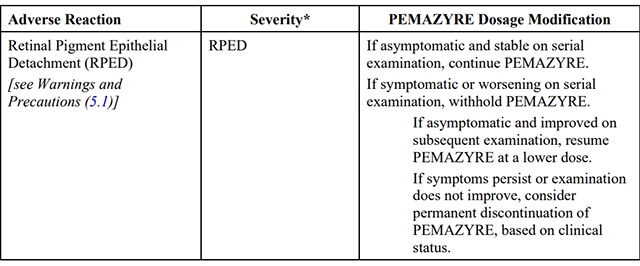
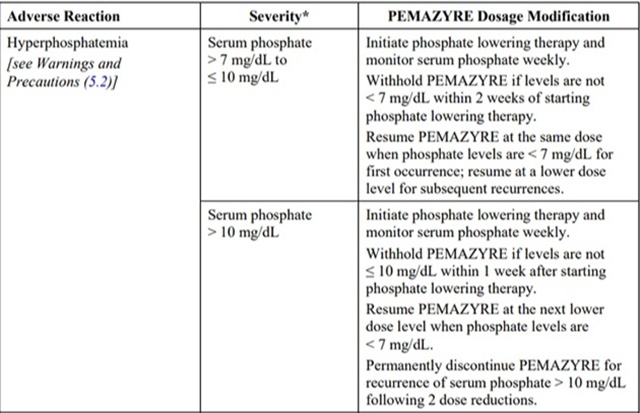
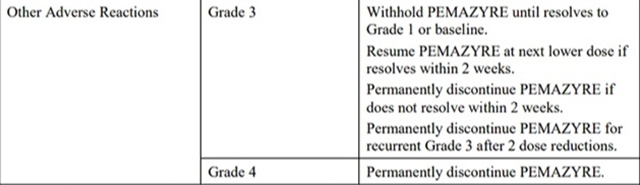
*Severity as defined by National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE)version 4.03.
Avoid concomitant use of strong and moderate CYP3A inhibitors with pemigatinib. If concomitant use with a strong or moderate CYP3A inhibitor cannot be avoided:
Reduce pemigatinib dosage from 13.5 mg to 9 mg.
Reduce pemigatinib dosage from 9 mg to 4.5 mg.
concomitant use of a strong or moderate CYP3A inhibitor is discontinued, increase the pemigatinib dosage (after 3 plasma half-lives of the CYP3A inhibitor) to the dosage that was used before starting the strong or moderate inhibitor.
The recommended dosage of pemigatinib for patients with severe renal impairment (eGFR estimated by Modification of Diet in Renal Disease [MDRD] 15 mL/min/1.73 m2 to 29 mL/min/1.73 m2) is 9 mg with the schedule (intermittent or continuous) designated for the indication.
The recommended dosage of pemigatinib for patients with severe hepatic impairment (total bilirubin > 3 × ULN with any AST) is 9 mg with the schedule (intermittent or continuous) designated for the indication.
from FDA,2022.08
As a potent and selective oral inhibitor targeting FGFR subtypes 1/2/3, Pemigatinib provides a new treatment option for cholangiocarcinoma patients.What is the dosage of Pemigatini···【more】
Article source:Lucius LaosRelease date:2024-08-13Recommended:196
Pemigatinib, brand name Damitan, is a potent and selective oral inhibitor against FGFR subtypes 1/2/3.How to use PemigatinibPemigatinibtablets should be swallowed whole, not chewed···【more】
Article source:Lucius LaosRelease date:2024-08-13Recommended:185
Pemigatinibis a small molecule kinase inhibitor targeting the fibroblast growth factor receptor (FGFR), primarily for the treatment of advanced or metastatic cholangiocarcinoma cau···【more】
Article source:Lucius LaosRelease date:2024-08-13Recommended:276

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
