

The efficacy of Entrectinib was evaluated in a pooled subgroup of patients with ROS1-positive metastatic NSCLC who received Entrectinib at various doses and schedules (90% received Entrectinib 600 mg orally once daily) and were enrolled in one of three multicenter, single-arm, open-label clinical trials: ALKA, STARTRK-1 (NCT02097810) and STARTRK-2 (NCT02568267).
Among the 92 patients, 10 had measurable CNS metastases at baseline as assessed by BICR and had not received radiation therapy to the brain within 2 months prior to study entry. Responses in intracranial lesions were observed in 7 of these 10 patients.
The efficacy of Entrectinib was evaluated in a pooled subgroup of adult patients with unresectable or metastatic solid tumors with a NTRK gene fusion enrolled in one of three multicenter, single-arm, open-label clinical trials: ALKA, STARTRK-1 (NCT02097810) and STARTRK-2 (NCT02568267).
Among the subset of patients who received prior systemic therapy for metastatic disease, the ORR was 53%, similar to that seen in the overall population.
Among the 54 adult patients, 4 had measurable CNS metastases at baseline as assessed by BICR and had not received radiation therapy to the brain within 2 months of study entry. Responses in intracranial lesions were observed in 3 of these 4 patients.
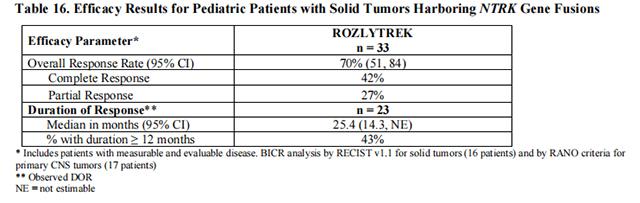
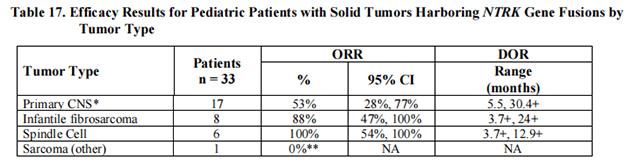
The efficacy of Entrectinib was evaluated in pediatric patients with unresectable or metastatic solid tumors with a NTRK gene fusion enrolled in one of two multicenter, open-label clinical trials: STARTRK-NG (NCT02650401) and TAPISTRY (NCT04589845).
Efficacy results are summarized in Tables 16 and 17.

from FDA,2024.01

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
