

The efficacy of Crizotinib for the treatment of patients with ALK-positive metastatic NSCLC, who had not received previous systemic treatment for advanced disease, was demonstrated in a randomized, multicenter, open-label, active-controlled study (Study 1).
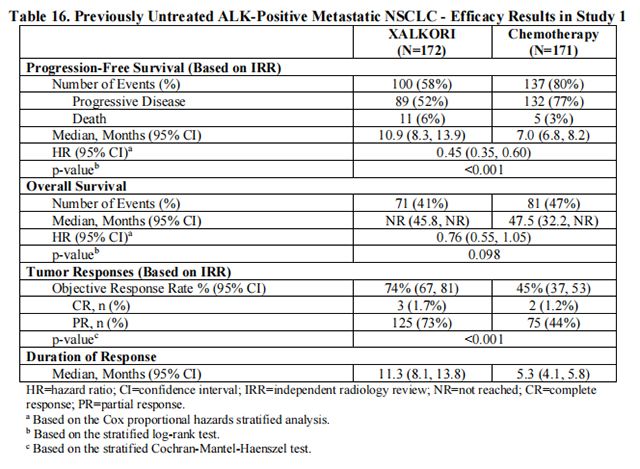
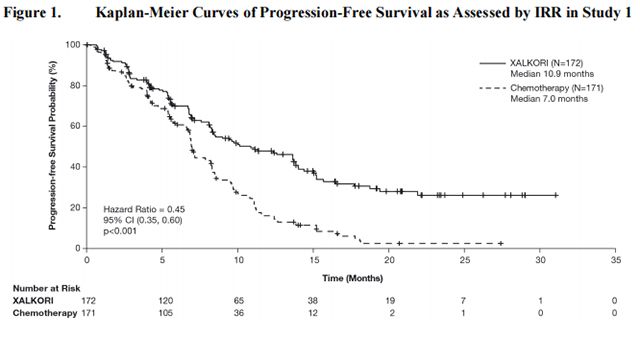
Study 1 demonstrated a statistically significant improvement in PFS in patients treated with Crizotinib. There was no statistically significant difference in OS between patients treated with Crizotinib and patients treated with chemotherapy. Table 16 and Figure 1 summarize the efficacy results. Exploratory patient-reported symptom measures of baseline and post-treatment dyspnea, cough, and chest pain suggested a delay in time to development of or worsening of dyspnea, but not cough or chest pain, in patients treated with Crizotinib as compared to chemotherapy. The patient-reported delay in onset or worsening of dyspnea may be an overestimation because patients were not blinded to treatment assignment.
from FDA,2023.09
Assessments of the medical value of specific treatment options need to be based on multidimensional chains of evidence.Does crizotinib workThe drug works by targeting aberrant prot···【more】
Article source:Lucius LaosRelease date:2025-04-03Recommended:242
The effect of treatment is a key indicator for evaluating the drug.Is crizotinib effective?According to the results of the phase III clinical trial, the median progression-free sur···【more】
Article source:Lucius LaosRelease date:2025-04-03Recommended:201

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
