

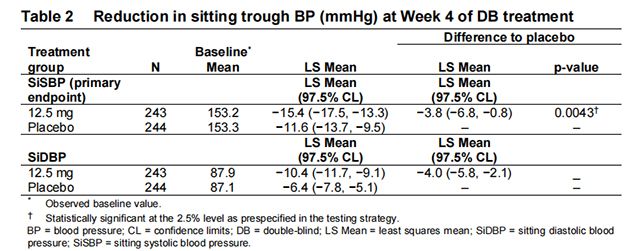
BP reductions compared to placebo based on uAOBP measurements at trough are shown in Table 2. Aprocitentan 12.5 mg was statistically superior to placebo in reducing SiSBP at Week 4 (part 1). The treatment effect was consistent for sitting diastolic BP (SiDBP) (Table 2)
The persistence of the BP-lowering effect of Aprocitentan was demonstrated in part 3 of the trial, in which patients on aprocitentan were re-randomized to placebo or 25 mg aprocitentan following a period during which all patients were treated with 25 mg. In patients re-randomized to placebo, the mean SiSBP increased, whereas in patients re-randomized to 25 mg aprocitentan the mean effect on SiSBP was maintained and was statistically superior to placebo at Week 40. The treatment effect was consistent for SiDBP.
Most of the BP-lowering effect occurred within the first two weeks of treatment with Aprocitentan. Aprocitentan is not approved for use at a 25 mg dose. The 25 mg dose has not demonstrated a meaningful improvement in blood pressure reduction as compared to the 12.5 mg dose and had an increased risk of edema/fluid retention.
Aprocitentan’s BP-lowering effect appeared consistent among subgroups defined by age, sex, race, BMI, baseline eGFR, baseline UACR, medical history of diabetes, and between BP measurement methodologies (uAOBP and ambulatory BP measurements).
from FDA,2024.03

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
