

The safety and efficacy of Fidaxomicin in pediatric patients 6 months to less than 18 years of age was investigated in a Phase 3, multicenter, investigator-blinded, randomized, comparative trial (NCT02218372). In this trial, 148 patients were randomized, of whom 142 received either Fidaxomicin or vancomycin in a 2:1 ratio. Randomized patients were stratified by age group as follows: 30 aged 6 months to <2 years, 49 aged 2 to <6 years, 40 aged 6 to <12 years, and 29 aged 12 to <18 years (one patient <6 months of age was enrolled in the trial). Treatment arms were balanced regarding demographics and other baseline characteristics.
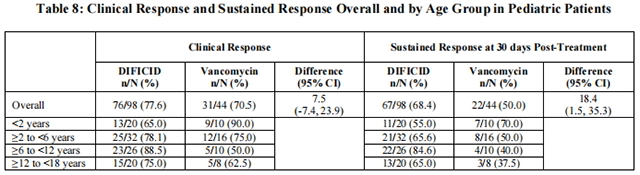
Clinical response for patients <2 years of age was defined as the absence of watery stools for at least 2 consecutive days while on treatment and the patient remained well with no requirement for further CDAD therapy through 2 days after completing treatment as assessed by the Investigator. Clinical response for patients ≥2 to <18 years of age was defined as <3 unformed bowel movements for at least 2 consecutive days while on treatment and the patient remained well with no requirement for further CDAD therapy through 2 days after completing treatment as assessed by the Investigator. Sustained clinical response was defined as the proportion of treated patients with confirmed clinical response and no CDAD recurrence through 30 days after end of treatment. The clinical response and sustained clinical response overall and by age groups are presented in Table 8.
FDA,2020.05
Fidaxomicin is an antibiotic carefully developed by Optimer to treat refractory Clostridium infections and has demonstrated excellent efficacy and safety through a unique mechanism···【more】
Article source:Lucius LaosRelease date:2024-08-29Recommended:258
Fidaxomicinis an innovative macrolide antibiotic that has demonstrated its potent therapeutic ability against refractory Clostridium infections by blocking bacterial RNA polymerase···【more】
Article source:Lucius LaosRelease date:2024-08-29Recommended:266
Fidaxomicinis a new star of macrolide antibiotics developed by Optimer, which has a specific effect on refractory Clostridium infection and has demonstrated excellent therapeutic p···【more】
Article source:Lucius LaosRelease date:2024-08-29Recommended:251

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
