

Previously Treated with Anti-angiogenic Therapy
The efficacy of Cabozantinib was evaluated in METEOR (NCT01865747), a randomized (1:1), open-label, multicenter trial of Cabozantinib versus everolimus conducted in patients with advanced RCC who had received at least 1 prior anti-angiogenic therapy. Patients had to have a Karnofsky Performance Score (KPS) ≥ 70%. Patients were stratified by the number of prior VEGFR tyrosine kinase inhibitors (TKIs) and Memorial Sloan Kettering Cancer Center (MSKCC) Risk Group.
Patients were randomized to receive Cabozantinib (N=330) 60 mg orally once daily or everolimus (N=328) 10 mg orally once daily. The majority of the patients were male (75%), with a median age of 62 years. Sixty-nine percent (69%) received only one prior anti-angiogenic therapy. Patient distribution by MSKCC risk groups was 46% favorable (0 risk factors), 42% intermediate (1 risk factor), and 13% poor (2 or 3 risk factors). Fifty-four percent (54%) of patients had 3 or more organs with metastatic disease, including lung (63%), lymph nodes (62%), liver (29%), and bone (22%).
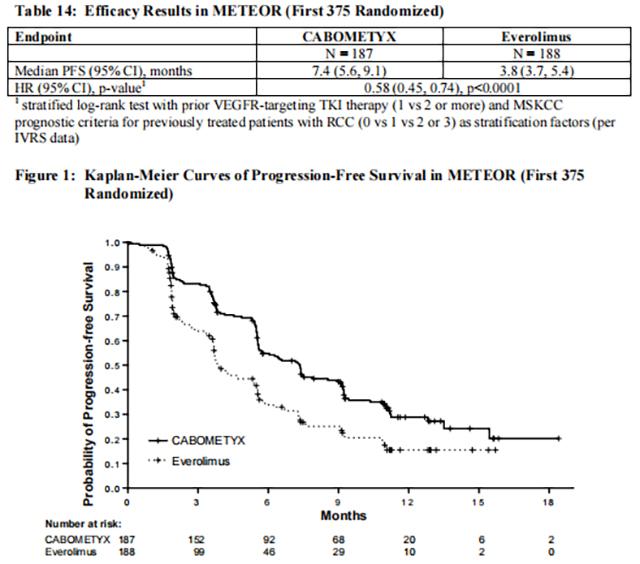
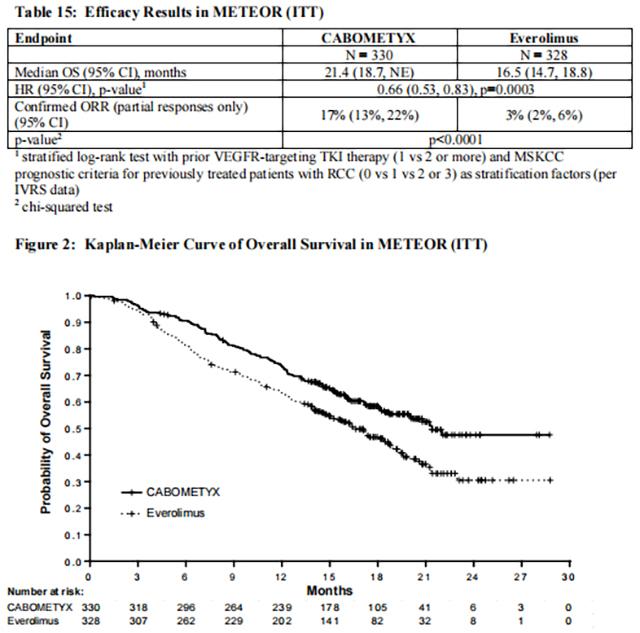
The main efficacy outcome measure was progression-free survival (PFS) assessed by a blinded independent radiology review committee among the first 375 subjects randomized. Other efficacy endpoints were objective response rate (ORR) and overall survival (OS) in the Intent-to-Treat (ITT) population. Tumor assessments were conducted every 8 weeks for the first 12 months, then every 12 weeks thereafter. Patients received treatment until disease progression or experiencing unacceptable toxicity. Patients on both arms who had disease progression could continue treatment at the discretion of the investigator.
Statistically significant improvements in PFS, OS, and ORR were demonstrated for Cabozantinib compared to everolimus. Efficacy results are presented in Tables 14 and 15 and Figures 1 and 2.
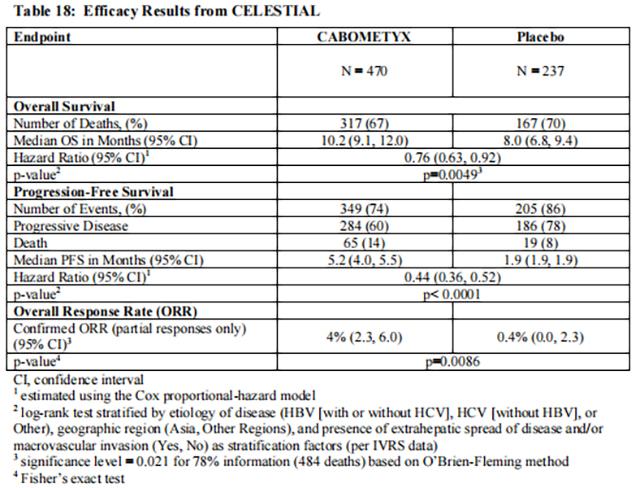
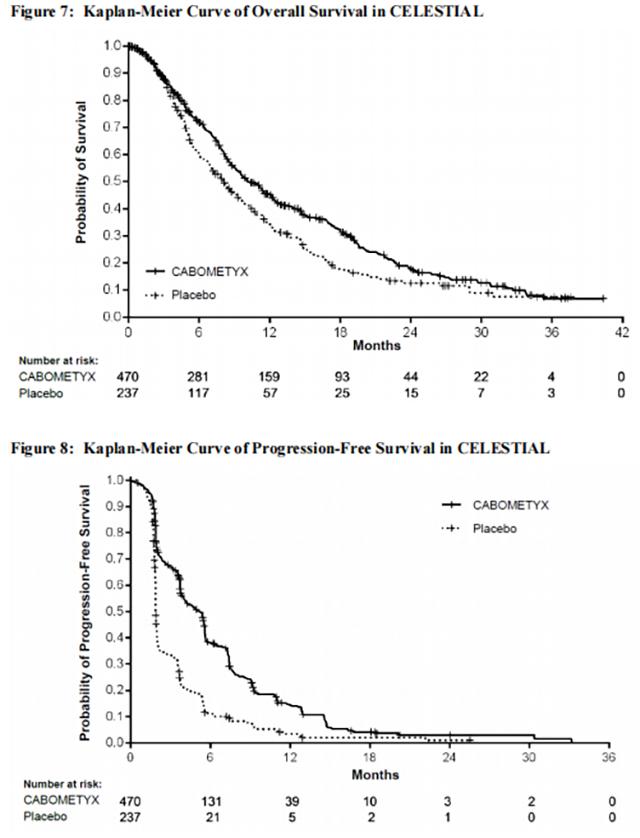
The efficacy of Cabozantinib was evaluated in CELESTIAL (NCT01908426), a randomized (2:1), double-blind, placebo-controlled, multicenter trial in patients with hepatocellular carcinoma (HCC) who had previously received sorafenib and had Child Pugh Class A liver impairment. Patients were randomized to receive Cabozantinib 60 mg orally once daily or placebo until disease progression or unacceptable toxicity. Randomization was stratified by etiology of disease (hepatitis B virus [HBV] with or without hepatitis C virus [HCV] vs. HCV [without HBV] vs. other [without HBV and HCV]), geographic region (Asia vs. other regions), and presence of extrahepatic spread of disease and/or macrovascular invasion (yes vs. no). The primary efficacy outcome measure was overall survival (OS). Additional outcome measures were progression-free survival (PFS) and objective response rate (ORR), as assessed by investigators per RECIST 1.1. Tumor assessments were conducted every 8 weeks.
In CELESTIAL, a total of 707 patients were randomized, 470 to Cabozantinib and 237 to placebo. The median age was 64 years (range 22 to 86 years), 82% were male, 56% were White and 34% were Asian. Baseline ECOG performance status was 0 (53%) or 1 (47%). The etiology of HCC was attributed to HBV in 38% of patients and HCV in 21%; etiology was attributed to causes other than HBV or HCV in 40%. Macroscopic vascular invasion or extra-hepatic tumor spread was present in 78% of patients and 41% had alpha-fetoprotein (AFP) levels ≥ 400 mcg/L. All patients received prior sorafenib and 27% received two prior systemic therapy regimens.
Efficacy results are summarized in Table 18, Figure 7, and Figure 8.
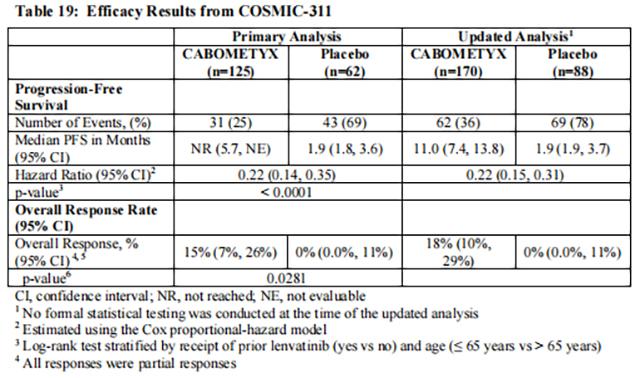
The efficacy of Cabozantinib was evaluated in COSMIC-311 (NCT03690388), a randomized (2:1), double-blind, placebo-controlled, multicenter trial in patients with locally advanced or metastatic differentiated thyroid cancer (DTC) that had progressed following prior VEGFR-targeted therapy and were radioactive iodine-refractory or ineligible. Patients were randomized to receive Cabozantinib 60 mg orally once daily or placebo with supportive care until disease progression or unacceptable toxicity. Randomization was stratified by prior receipt of lenvatinib (yes vs. no) and age (≤ 65 years vs > 65 years). Eligible patients randomized to placebo were allowed to cross-over to Cabozantinib upon confirmation of progressive disease by blinded independent radiology review committee (BIRC). The multiple primary efficacy outcome measures were progression-free survival (PFS) in the ITT population, and overall response rate (ORR) in the first 100 randomized patients, as assessed by BIRC per RECIST 1.1. Tumor assessments were conducted every 8 weeks. Overall survival (OS) was a descriptive outcome measure.
The primary analysis of PFS included 187 randomized patients. An updated analysis of PFS was performed and included 258 randomized patients. The median age was 65 years (range 31 to 85 years), 53% were female, 70% were White, 19% were Asian, 2% were Black, 2% were American Indian or Alaska Native, and 63% received prior lenvatinib. Baseline ECOG performance status was 0 (46%) or 1 (54%) and 93% of patients had metastatic disease.
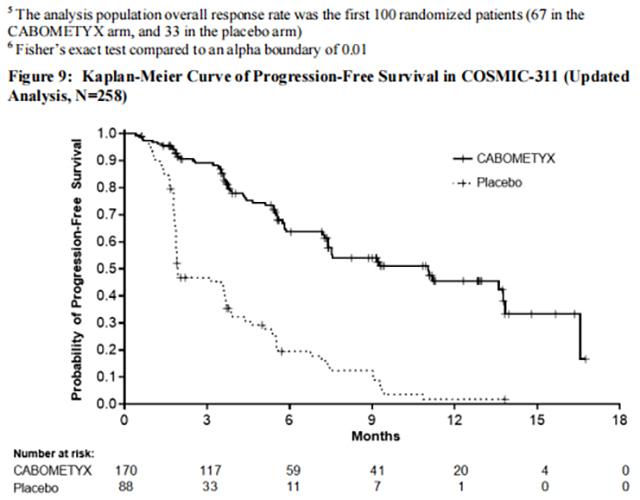
The trial demonstrated a statistically significant improvement in PFS, while it failed to demonstrate a statistically significant improvement in ORR, for patients randomized to Cabozantinib compared with placebo. Efficacy results are summarized in Table 19 andFigure 9.
from FDA,2023.09
Cabozantinib is a multi-targeted tyrosine kinase inhibitor that has attracted much attention in the field of oncology therapy.Does cabozantinib workThe clinical effect of cabozanti···【more】
Article source:Lucius LaosRelease date:2025-02-20Recommended:230
Cabozantinib is a multi-targeted tyrosine kinase inhibitor that plays an important role in cancer treatment, and its therapeutic efficacy has attracted much attention.How effective···【more】
Article source:Lucius LaosRelease date:2025-02-19Recommended:208
Cabozantinib is a multi-targeted tyrosine kinase inhibitor that has demonstrated unique efficacy in cancer treatment, and its therapeutic effect has attracted much attention.Is cab···【more】
Article source:Lucius LaosRelease date:2025-02-19Recommended:212

Lucius Pharmaceutical Co., Ltd., was established in 2020 in Vientiane, the capital of Laos. It aims to offer safe, effective, and affordable medicines globally. With a factory spanning 25,000 square meters, the company manufactures 200+ generic drugs in diverse therapeutic fields.
Address:No.26 Thongmang village, Xaythany district, Vientiane Capital, Laos
E-mail:laoslucius@gmail.com
Whatsapp:
